Neuralink in 2024: The Year of Noland Arbaugh, the First Human User of the N1 Chip
From Neuralink’s perspective, 2024 could be called the year of Noland Arbaugh. The company’s clinical team really had a lucky hand in choosing the first candidate. It’s hard to imagine a better applicant. Noland is very open about everything related to the interface and his life. He is a natural speaker and has no problem with the media. He gives a lot of interviews and is active on social media. It’s all just the greatest PR for Neuralink.
The first N1 implant in a human was by far the most significant event of the year for Neuralink. However, during the year, the company also managed to implant the interface a second time, received approval for tests in Canada and significantly advanced preparations for testing of the new visual prosthesis. In this article, we summarize the most important milestones of the year and try to predict what we may see in 2025.
Milestones of 2024
The year 2024 will forever be remembered in Neuralink’s history as the year of the first human implantation of its neural interface. However, there have also been a number of other significant milestones.
January 28th, 2024: The first person received the Neuralink interface
Neuralink finally implanted its neural interface into a human user for the first time, less than eight years after its founding in 2016. Elon Musk announced it in X post:
The first human received an implant from @Neuralink yesterday and is recovering well.
Initial results show promising neuron spike detection.
— Elon Musk (@elonmusk) January 29, 2024
As it turned out, the first patient was Noland Arbaugh, a 29-year-old quadriplegic from Yuma, Arizona, former Texas A&M student. Despite his disability, Noland is very open, has a brilliant sense of humor and is slowly becoming an Internet sensation having almost 130k followers on X.
March 2024: Electrode retraction problem
A few weeks after the surgery, a problem with the implanted electrode threads occurred. Most of them became loose and pulled away from Noland’s brain. Only about 15% of the electrodes remained active. This temporarily degraded the implant’s performance. However, in response to this change, Neuralink specialists:
- modified the recording algorithm to be more sensitive to signals from groups of neurons
- improved the way these signals are converted into cursor movements
- improved the user interface of Neuralink application
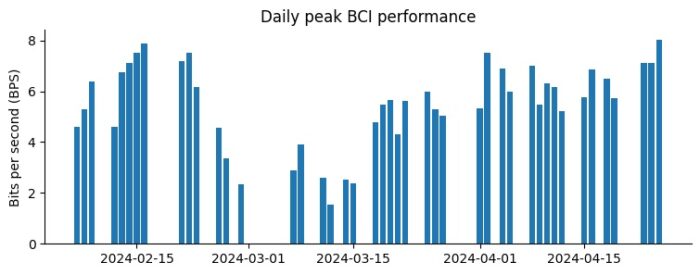
These optimizations brought rapid and sustained improvements in interface performance, and today Noland is once again breaking his previous interface throughput records.
July 10th, 2024: Unexpected Neuralink presentation
Neuralink gave a presentation on X showing plans how to resolve the situation with electrode threads retractions in further users. Elon Musk announced that the company is already preparing for a second implantation in a human user and that several more patients should use the interface by the end of the year 2024. In the second part of the live broadcast, the participants also answered several questions from X users.
— Neuralink (@neuralink) July 10, 2024
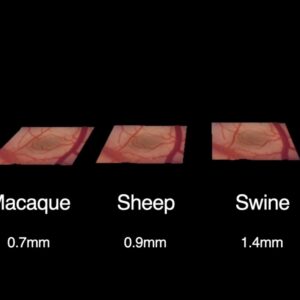
In the main part of the presentation, Neuralink representatives showed presumed causes of the electrode threads’ retractions in the first study participant and several ways in which they intend, without any change to the current hardware, to prevent the threads from pulling out of the brain in other users. Neuralink specialists saw the main reason for retractions as greater than expected movement of the brain in the skull. Among the measures to eliminate the problem were:
- reduction of air pockets that form in the skull after surgery
- removal of the empty space between the implant and the brain
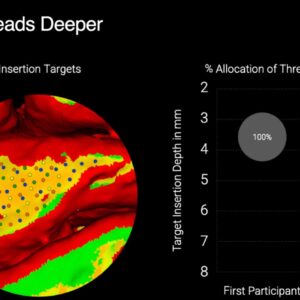
- implantation of the electrode threads deeper into the brain
- Credit: Neuralink
- Credit: Neuralink
- Credit: Neuralink
- Credit: Neuralink
July 2024: Second N1 user
A second patient named Alex was given the Neuralink interface sometime in late July 2024. However, he is the opposite of Noland in terms of public openness, and we haven’t learned much about him yet.
Thanks to a post on the Neuralink blog, we know that he is a man. He is a quadriplegic, meaning he cannot move any of his limbs. He is probably around 25-35 years old. He likes to play computer games, specifically Counter Strike 2. His surgery was also performed at the Barrow Neurological Institute in Phoenix, Arizona. He has one Neuralink implant, probably in the motor area of the brain, specifically in the part responsible for the movements of one of his hands.
There were no complications during and after Alex’s surgery. He left the hospital the next day and recovered without problems. Thanks to Elon Musk’s interview with Lex Fridman, we also know that Alex’s implant has around 400 active electrodes, which Elon says is a good result. Once he learned how to operate the interface, he started working with the Fusion 360 CAD software and even designed his own interface charger holder, which was then 3D printed and integrated into his special computer desk. Here’s a video of Alex working with the CAD software:
September 2024: Breakthrough Device Designation for Blindsight
The US Food and Drug Administration (FDA) has designated Neuralink’s upcoming vision prosthesis, called Blindsight, as a “Breakthrough Device”. This is the first significant step toward approval for an initial clinical trial. The Breakthrough Devices Program should provide Neuralink with an acceleration of the FDA approval process.
We have received Breakthrough Device Designation from the FDA for Blindsight.
Join us in our quest to bring back sight to those who have lost it. Apply to our Patient Registry and openings on our career page https://t.co/abBMTdv7Rh
— Neuralink (@neuralink) September 17, 2024
Blindsight, a vision prosthesis, is supposed to be Neuralink’s second product after “Telepathy”, an interface controlling computers and other devices using just thoughts. Blindsight should provide vision even for the people who have been blind since birth. At first with low resolution, later getting much better, and eventually even in various electromagnetic spectra, similar to Jordi La Forge from the series Star Trek: The Next Generation.
November 2024: Permission for testing in Canada
A year and a half after receiving approval for human testing in the US, Neuralink has been granted permission to test in Canada. Health Canada, the agency responsible for Canadian federal health policy, granted Neuralink a clinical trial permit.
🇨🇦 We’re happy to announce that Health Canada has approved the launch of our first clinical trial in Canada! Recruitment is now open.
If you have quadriplegia due to ALS or SCI, you may qualify. Visit our Patient Registry to learn more and apply.https://t.co/5BySJABkkO
— Neuralink (@neuralink) November 20, 2024
The trials will be conducted at the Krembil Brain Institute of The University Health Network (UHN) in Toronto. The study will have three objectives, as in the US:
- confirmation of N1 interface security
- confirmation of the safety of the R1 robot and the interface implantation process
- confirmation of the functionality of the implant for controlling a computer and other external devices
November 2024: Permission for robotic arm control tests
Neuralink has received official permission to test controlling robotic arms. The N1 interface, which already has two users, will be used not only to control computers, but also robotic upper limbs.
https://twitter.com/neuralink/status/1861107594645119006
Neuralink may use Tesla’s Optimus robot arm for these tests. The question is how many movements can be mapped to the N1 interface. The latest version of the Optimus hand has 22 degrees of freedom. This is what the hand looked like at the We, Robot presentation, where the Tesla Cybercab autonomous vehicle was introduced:
What can we expect in 2025?
Let’s try to predict what Neuralink could bring next year:
How many patients by the end of 2025?
Neuralink planned to have 10-11 patients by the end of 2024. It probably didn’t happen, unless the company is hiding something. We know for sure about two users. The 10-11 patients mentioned could be real by the end of 2025.
Today, the table of Neuralink interface users looks like this:
| # | Name: | Gender: | Age at Implantation: | Implantation Date: | Implantation Site: | Clinic: | Device type: | Number of Implants: | Cerebral Hemisphere: | Brain Area: |
| 1 | Noland Arbaugh | male | 29 | January 28, 2024 | Phoenix, Arizona | Barrow Neurological Institute | N1 Telepathy | 1 | left | motor cortex, right hand area |
| 2 | Alex | male | — | late July 2024 | Phoenix, Arizona | Barrow Neurological Institute | N1 Telepathy | 1 | — | — |
First patients in Canada
That’s quite likely. Permission for testing was granted. The clinic has been chosen. The patient registry in Canada has been operating for almost a year. All that’s left is to select suitable candidates and perform surgery.
Permission for testing in the UK
This might seem quite likely, given that UK patient registry has already been established. However, politics may come in play. The current British government does not really like Elon Musk, to put it mildly.
Breakthrough Device designation for an interface bridging a damaged spinal cord
Spinal cord bridging is expected to be the third Neuralink’s product. The company has been working on it for several years. The first step towards human testing will be, as with the “Telepathy” and “Blindsight”, the FDA’s “Breakthrough Device” designation. It may possibly come next year.
The first Blindsight implanted?
This probably won’t happen next year. More likely in 2026.